The concept and design of a manufacturing system using Spectrum's hollow fiber modules for applications with specific requirements must be conducted through intensive consultation and discussion with Spectrum's production management and process application specialists. A design engineer assures that specific customer requirements are compatible and are incorporated into the constraints of the system design.
 A team of specialized engineers (mechanical, automation, validation, etc.) all contribute to the creation of the manufacturing filtration system in an ISO 9000 certified environment, complying with cGMP regulations. Incorporating the customer's specific requirement, detailed engineering drawings of the system are then issued for the customer's approval. In addition, validation protocols for the Installation Qualification and the Operation Qualification are generated and executed in accordance and approval by the customer's QC department. Required automation is designed and engineered according to cGMP and 21CFR part 11 regulations. A Factory Acceptance Test (FAT) according to an approved protocol finalizes this process.
A team of specialized engineers (mechanical, automation, validation, etc.) all contribute to the creation of the manufacturing filtration system in an ISO 9000 certified environment, complying with cGMP regulations. Incorporating the customer's specific requirement, detailed engineering drawings of the system are then issued for the customer's approval. In addition, validation protocols for the Installation Qualification and the Operation Qualification are generated and executed in accordance and approval by the customer's QC department. Required automation is designed and engineered according to cGMP and 21CFR part 11 regulations. A Factory Acceptance Test (FAT) according to an approved protocol finalizes this process.
Installation:
After the FAT is successfully completed, the filtration system will then be prepared for shipment to the customer. After assurance of necessary facility utilities, our specialized service engineers will perform the Site Acceptance Test (SAT) according to a pre-approved SAT protocol. During the SAT, operator training is conducted on the new filtration system. Once the SAT is successfully completed, the system will then be turned over to the customer for manufacturing operation.
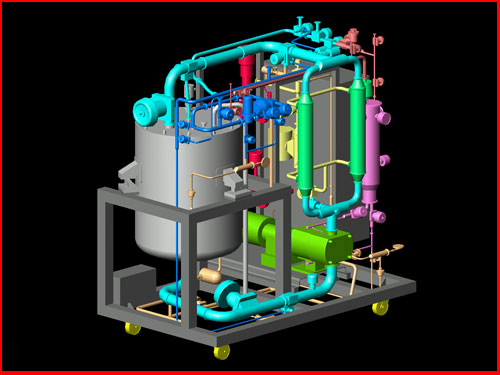
The custom system configuration depends on the process specifications and desired operation as deemed by the customer. Since most biopharmaceutical processes involve sensitive biological components, sanitary rotary lobe pumps are used for the circulation required for the gentle filtration achieved with Spectrum's hollow fiber tangential flow membranes. System flow control can include diaphragm shut-off valves, and automated sanitary valves to control and regulate circulation and filtrate flows as well as back pressures and TMP.
In addition to pressure transducers and flow meters, optional equipment can be installed such as temperature sensors, conductivity meters, pH probes and mass-flow controllers. The system can also be equipped with different types of processing reservoirs with or without temperature control jacketing. The extent of automation can range from total manual operation to complete system automation with full data acquisition and SCADA process control systems.





